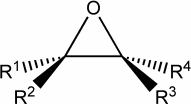
Chiral molecules are optically active :
Starting with heptane, the possibility exists that a single skeleton
corresponds to several spatial configurations.
In particular, this happens whenever the molecule includes just
one so-called chiral carbon,
namely a carbon atom bonded to 4 different
ligands.
In that case, we are faced with a
chiral compound
with two different possible configurations which are mirror images of each other
(they are called enantiomers).
As it is traversed by a ray of polarized light,
a pure enantiomer in fluid form (or in a solution) will rotate
the angle of polarization by a angle proportional to the molar density and the distance
travelled.
Such an optical activity
is observed for the following two types of heptane.
Each of these has two enantiomers because each has a single chiral atom :
The fact that either of those yields a pair of enantiomers is the reason why there are
11 stereoisomers of heptane for only 9 structural isomers.
It's often
the case that a molecule with k chiral carbons
has 2k stereoisomers.
The simplest exception among alkanes is the following octane,
featuring two chiral carbons but only
3 (not 4) stereoisomers; a pair of
optically active enantiomers and one inactive
meso compound.
(HINT: As the two halves may rotate around the axis of
the two chiral carbons, the meso isomer is center-symmetric.)
3,4-Dimethylhexane =
( C* H CH3 C2H5 ) 2
A star superscript ( C* )
is used to indicate that a given carbon is chiral.
On the limited usefulness of the "chiral carbon" concept :
Spiranes are cycloalkanes which contain two cycles that share a
single carbon atom. At that central atom, the two pairs
of bonds that define the planes of the two cycles are perpendicular.
The simplest example of a spirane is spiroheptane,
which consists of 2 carbon quadrilaterals sharing one vertex.
Spiranes can illustrate some of the difficulties associated with
chiral carbons in the analysis of delicate cases.
For example, consider the following pair of enantiomers
for dimethylspiroheptane
C9H16
This is clearly a
chiral compound because those two mirror images cannot be superposed.
Such molecules are sometimes wrongly heralded as having
no chiral carbons.
A close examination reveals that this is not the case; the above chiral
molecule does feature 3 chiral carbons (the central carbon and the two
carbons attached to methyl groups).
Indeed, a carbon is chiral whenever it's attached to
4 different ligands. Two chiral ligands that are enantiomers of each
other are different!
When two ligands are interconnected by a structurally symmetrical chain,
the case may not be obvious to settle.
In the case of a carbon attached to a methyl group in the aforementioned molecule of
dimethylspiroheptane, the chain that goes from
one bond to the other and the chain that goes back have different chiralities
(otherwise the whole molecule would not be chiral). Both of those carbons
are therefore chiral.
The case of the central atom is even trickier.
It belongs to two oriented 4-cycles which are symmetrical but chiral
(we may decide to observe from the side of the methyl group and describe unambiguously
a direction as either clockwise or counterclockwise).
Some thinking is needed to realize that two
identical chiral loops meeting perpendicularly at one point form a chiral
configuration (the two chirality do not cancel, so to speak).
The central carbon is thus chiral as well.
Removing an hydrogen atom from an alkane yields an active
chemical entity called an alkyl group
(it's eager to combine with some other "free" group,
as the two unpaired electrons from both groups tend to form a
covalent bond).
As already illustrated above, such groups are commonly
named after the
simple alkane they are derived from (by removing an hydrogen from
a carbon atom at the end of a chain):
Methyl, ethyl, propyl, butyl, etc.
-CH3
-C2H5
-C3H7
-C4H9 ...
In the standard nomenclature used to describe "branched" alkanes,
the longest carbon chain
is used along with the names and numeric positions of the akyl groups borne
by carbons on that chain. Symmetries are usually taken advantage of,
in order to make the numeric positions as small as possible.
For example, a descriptive name for isobutane is
methylpropane: A methyl group attached to the middle atom
(position 2) in the 3-chain of propane...
The position is not explicited in this case because there's
only one possibility which does not yield a compound with a
simpler name (namely, straight butane ).
Several akyl groups may be attached to the same carbon atom.
For example, dimethylpropane properly describes a pentane
(also called neopentane)
consisting of a central carbon atom attached
to 4 identical methyl groups.
Wikipedia :
Alkanes
|
Cubane
|
Dodecahedrane
|
Cahn-Ingold-Prelog convention
The simplest aromatic compound is benzene
C6H6 whose cyclic structure was
first proposed in 1865 by
August Kekulé (1829-1896)
the father of structural chemistry.
A Phenyl -C6H5
(symbol Ph, formerly F) consists of six carbons
in a circle, all bonded to an hydrogen with at most one exception
(no exception in the case of benzene, which may be denoted by the formula PhH).
All six carbon atoms are equally spaced, regardless of their positions with
respect to the substituent.
Toluene (PhCH3) is also known as phenyl methane.
as it consists of a phenyl group and a methy group, the IUAPC recommends the systematic name methylbenzene.
Phenol (PhOH). Also called carbolic acid.
It consists of a phenyl group and an hydroxyl group (-OH).
As such, it's also known as phenyl hydroxide.

Gum benzoin
|
Beinzoic acid
|
Benzene
|
Phenyl (Ph)
|
Aromatic
|
Cahn-Ingold-Prelog convention